| Generic Drug/Consistency Evaluation Platform |
Services and Solutions
Fukangren is committed to the research and development of new drugs and the consistency evaluation of generic drugs. It has a strong team of highly educated core personnel with rich experience in new drug R&D and industrialization.
| High-end preparation platform |
| Innovative medicine/international cooperation platform |
| Clinical research and development platform |
| MAH Licensed Service Platform |


CDMO
Focus on the development of high-end preparations and international cooperation in...
| R&D Center |
| Industry Base |

| Project Management |
| Quality Assurance |

About Us
Beijing Fukangren Biopharmaceutical Technology Co., Ltd. started its drug R&D service in 1999 and is the CRO company with the longest continuous service time in China. Owns 3 pharmaceutical R&D centers, 1 clinical R&D center, 4 API production bases and 1 preparation production base
| About Us |
| Development Path |
| Company Culture |
| Company Team |
| Business Planning |


Contact Us
Sound organization, high-quality quality management team, complete document management system...
| Business Cooperation |
| Talent Recruitment |

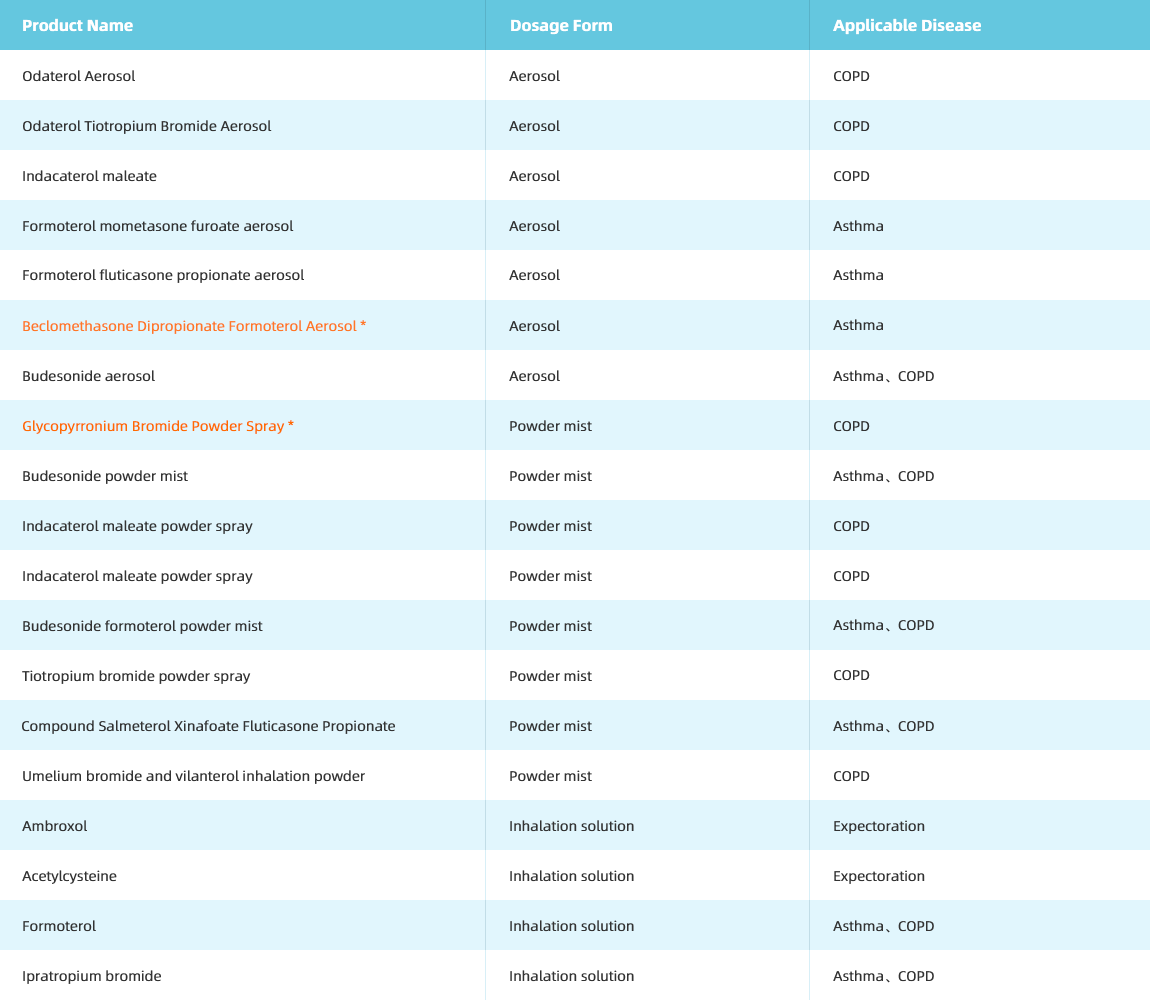
Inhalation solution/aerosol/powder mist
High-end preparation platform
Committed to research and development of new drugs and consistency evaluation of generic drugs

Technical Advantages

Familiar with the key process control points of various inhalation preparations, and the evaluation indexes of in vivo and in vitro consistency of inhalation preparations

Professional special formulation R&D team
Series Products
Equipment Advantages
Test equipment
The first domestic enterprise to develop inhalation gas/powder mist formulations research and development
The first domestic company to introduce the most advanced international equipment
Inhalation device
Joint research and development and introduction of powder aerosol inhalation device